重塑器官移植的技术
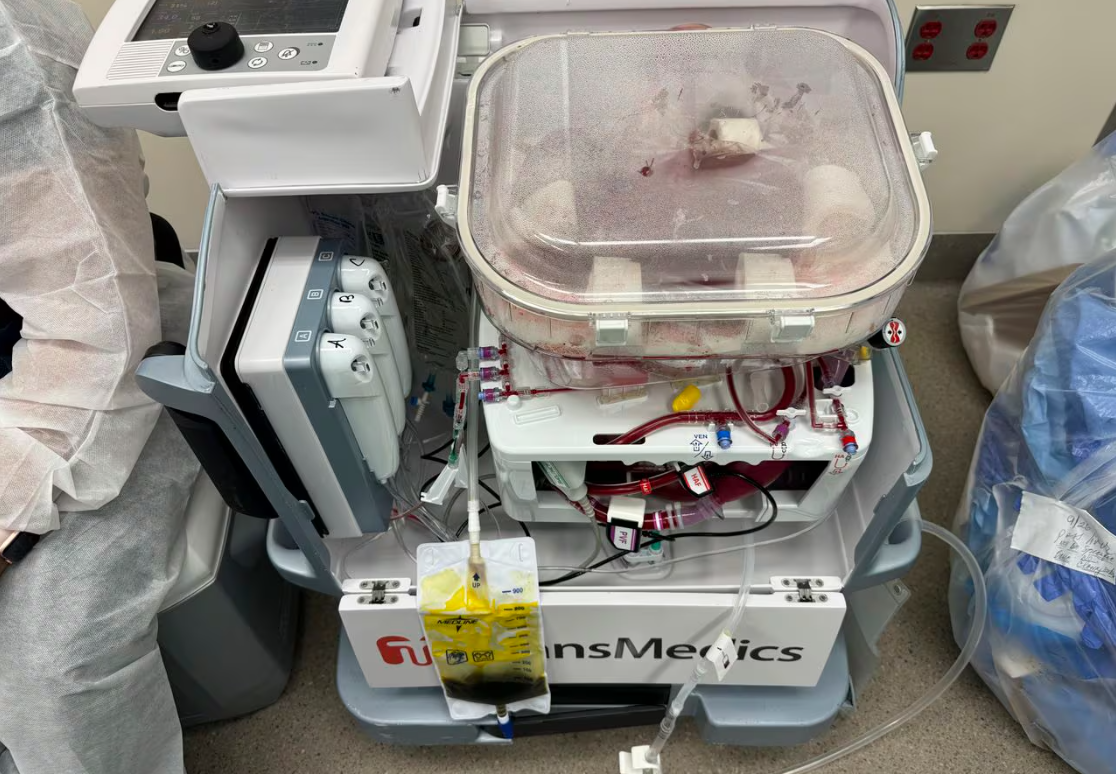
【中美创新时报2024 年 4 月 3 日编译讯】(记者温友平编译)长期以来,科学家们一直在试验各种技术,使器官保持在更动态的条件下,温度更高,并灌注血液或其他含氧溶液。经过多年的开发,第一个通过灌注保护肺部的设备于 2019 年获得美国食品和药物管理局的批准。用于灌注心脏和肝脏的设备于 2021 年底获得批准。《纽约时报》记者特德·奥尔康(Ted Alcorn)对此作了下述报道。
从某种程度上来说,芝加哥西北纪念医院手术室里的人类肝脏是活的。通过其组织循环的血液输送氧气并清除废物,该器官产生身体必需的胆汁和蛋白质。
但捐赠者已于一天前去世,肝脏躺在一个四四方方的塑料装置内。该器官的生命力归功于这台机器,它保存着它以便移植到有需要的患者体内。
“这有点像科幻小说,”该医院的移植外科医生丹尼尔·博尔哈-卡乔博士说。
外科医生正在对转基因动物的器官进行实验,暗示未来它们可能成为移植的来源。但在广泛使用的技术的推动下,该领域已经在经历范式转变,这些技术允许临床医生在体外暂时储存器官。
所谓的灌注正在改变器官移植过程的各个方面,从外科医生的操作方式,到可以捐献器官的患者类型,再到受体的结果。
最重要的是,采用灌注的外科手术正在移植更多的器官。
自 2020 年以来,西北大学的肝移植量增加了 30%。在全国范围内,肺、肝和心脏移植数量到 2023 年将分别增加 10% 以上,这是几十年来同比增幅最大的之一。
没有血液流动,器官会迅速恶化。这就是为什么临床医生长期以来一直认为理想的器官捐献者是在大脑活动终止的情况下死亡但心脏继续跳动的人,使器官保持活力,直到与接受者相匹配。
为了最大程度地减少器官在从捐献者的血液供应中取出后以及与接受者的血液连接之前对器官的伤害,外科医生常常将它们冷却到略高于冰点的温度,从而显着减慢它们的代谢过程。
这延长了器官移植的时间,但只是短暂的。肝脏的存活时间不超过 12 小时,肺和心脏的存活时间接近 6 小时。
长期以来,科学家们一直在试验各种技术,使器官保持在更动态的条件下,温度更高,并灌注血液或其他含氧溶液。经过多年的开发,第一个通过灌注保护肺部的设备于 2019 年获得美国食品和药物管理局的批准。用于灌注心脏和肝脏的设备于 2021 年底获得批准。
这些装置本质上是通过管道将血液或含氧液体泵入捐赠器官的血管中。 由于灌注器官中的细胞会继续发挥作用,临床医生可以更好地评估该器官是否会在受体体内茁壮成长。
佛罗里达州梅奥诊所的外科教授克里斯·克鲁姆博士说,在这些信息的支持下,移植外科医生已经开始使用年龄较大或病情较重的捐赠者的器官,否则他们可能会拒绝这些捐赠者。 “我们正在寻找以前从未有过的器官,并且我们看到了良好的结果,”他说。
也许最重要的是,灌注进一步为昏迷患者的器官捐献打开了大门,这些患者的家人已经撤回了生命支持,让他们的心脏最终停止跳动。每年,都有数以万计的人在循环停止后以这种方式死亡,但他们很少成为捐赠者的候选人,因为死亡过程剥夺了他们的器官的氧气。
现在,外科医生正在对这些器官进行灌注,要么将它们移至机器上,要么以技术含量较低的方式在捐献者身体的该区域进行血液再循环。这使得它们对移植更具吸引力。
根据运营美国移植系统的非营利组织器官共享联合网络的数据分析,自 2020 年以来,捐献者循环死亡后移植的肝脏数量增加了一倍。
曾经,外科医生从未使用过此类捐赠者的心脏,因为该器官对缺氧很敏感。2023 年,通过灌注,他们移植了 600 多个。
移植中心表示,通过利用这一新的捐赠者队伍,他们可以更快地为多余的急需患者找到器官。 希穆尔·沙阿博士表示,他在辛辛那提大学领导的器官移植项目基本上消除了肝脏等待名单。 “我从未想过,在我的职业生涯中,我会这么说,”他说。
采用该技术的一个障碍可能是成本。 按照设备制造商目前要求的价格,在体外灌注器官可能会使移植费用增加超过 65,000 美元; 较小的医院可能无法证明前期费用的合理性。
领先公司之一的 TransMedics 在监管机构批准其设备后大幅提高了价格,亚利桑那州共和党众议员保罗·戈萨尔 (Paul Gosar) 发出了一封严厉的信,信中写道:“一开始是一项有前途的医疗设备创新,也是增加全国移植的机会 现在被一家已经迷失了方向的上市公司扣为人质。”
但一些外科医生表示,该技术仍然可以节省资金,因为接受灌注器官的患者通常可以更快地出院,并发症更少,并且有更好的中长期结果。
外科医生仍在探索灌注器官在体外存活时间的上限,尽管技术已经在很大程度上改变移植,但有人说这只是一个开始。
多伦多大学外科医生沙夫·克沙维吉(Shaf Keshavjee)博士的实验室处于开发体外肺保存技术的最前沿,他表示,这些设备最终可以让医生切除、修复肺部,然后将其送回病人体内,而不是更换它们…… “我认为我们可以制造出比植入的受体寿命更长的器官,”他说。
范德比尔特大学 (Vanderbilt University) 是美国最繁忙的心脏移植项目之一,该大学心脏外科主任阿什什·沙阿 (Ashish Shah) 博士对此表示同意,并称其为“圣杯”。
“你的心很糟糕,”他说。 “我把它拿出来。我把它放在我的设备上。虽然你没有心脏,但我可以用人造心脏支撑你一段时间。 然后我取出你的心脏并修复它——细胞、线粒体、基因疗法等等——然后我把它缝回去。你自己的心脏。这才是我们真正努力的目标。”
本文最初发表于《纽约时报》。
题图:灌注机中装有已故捐赠者的肝脏。该机器本质上是将血液或含氧液体通过管道泵入捐赠器官的血管中。DANIEL BORJA-CACHO/NORTHWESTERN MEDICINE/NYT
The technique reshaping organ transplantation
By Ted Alcorn New York Times,Updated April 2, 2024
On some level, the human liver in the operating room at Northwestern Memorial Hospital in Chicago was alive. Blood circulating through its tissues delivered oxygen and removed waste products, and the organ produced bile and proteins that are essential to the body.
But the donor had died a day earlier, and the liver lay inside a boxy plastic device. The organ owed its vitality to this machine, which was preserving it for transplantation into a needy patient.
“It’s a little bit science fiction,” said Dr. Daniel Borja-Cacho, a transplant surgeon at the hospital.
Surgeons are experimenting with organs from genetically modified animals, hinting at a future when they could be a source for transplants. But the field is already undergoing a paradigm shift, driven by technologies in widespread use that allow clinicians to temporarily store organs outside the body.
Perfusion, as it is called, is changing every aspect of the organ transplant process, from the way surgeons operate, to the types of patients who can donate organs, to the outcomes for recipients.
Most significantly, surgical programs that have adopted perfusion are transplanting more organs.
Since 2020, Northwestern has had a 30 percent uptick in its volume of liver transplants. Nationally, the number of lung, liver, and heart transplants each rose by more than 10 percent in 2023, one of the largest year-over-year increases in decades.
Without blood flow, organs rapidly deteriorate. That’s why clinicians have long considered the ideal organ donor to be someone who died under circumstances that ended brain activity but whose heart continued beating, keeping the organs viable until they could be matched with recipients.
To minimize injury to organs after their removal from a donor’s blood supply and before they are connected to a recipient’s, surgeons used to cool them to just above freezing, significantly slowing their metabolic processes.
This extends the window in which organs can be transplanted, but only briefly. Livers remain viable for no longer than 12 hours, and lungs and hearts closer to six.
Scientists have long experimented with techniques for keeping organs in more dynamic conditions, at a warmer temperature and perfused with blood or another oxygenated solution. After years of development, the first device for preserving lungs via perfusion won approval from the Food and Drug Administration in 2019. Devices for perfusing hearts and livers were approved in late 2021.
The devices essentially pump blood or an oxygenated fluid through tubes into the blood vessels of the donated organ. Because cells in a perfused organ continue to function, clinicians can better assess whether the organ will thrive in a recipient’s body.
Bolstered by that information, transplant surgeons have begun to use organs from older or sicker donors that they might otherwise have turned down, said Dr. Kris Croome, a professor of surgery at the Mayo Clinic in Florida. “We’re going after organs we never would have before, and we’re seeing good outcomes,” he said.
Perhaps most important, perfusion has further opened the door to organ donation by comatose patients whose families have withdrawn life support, allowing their hearts to eventually stop. Each year, tens of thousands of people die this way, after the cessation of circulation, but they were rarely donor candidates because the dying process deprived their organs of oxygen.
Now, surgeons are perfusing these organs, either by removing them to a machine or, in a lower-tech manner, by recirculating blood in that region of the donor’s body. And that has made them much more appealing for transplant.
Since 2020, the number of livers transplanted after the circulatory death of the donor has doubled, according to an analysis of data from the United Network for Organ Sharing, the nonprofit that runs the United States’ transplant system.
Once, surgeons never used hearts from such donors because of that organ’s sensitivity to oxygen deprivation; in 2023, thanks to perfusion, they transplanted over 600.
By tapping this new cadre of donors, transplant centers said they could find organs more quickly for the excess of patients in urgent need. Dr. Shimul Shah said the organ transplant program he directs at the University of Cincinnati had essentially wiped out its waiting list for livers. “I never thought, in my career, I would ever say that,” he said.
One obstacle to the adoption of the technology may be cost. At the rates currently demanded by device makers, perfusing an organ outside the body can add more than $65,000 to the price of a transplant; smaller hospitals may not be able to justify the upfront expense.
One of the leading companies, TransMedics, raised its prices substantially after regulators approved its device, prompting a stern letter from Representative Paul Gosar, an Arizona Republican, who wrote: “What began as a promising medical equipment innovation and an opportunity to increase transplantation nationwide is now being held hostage by a public company that has lost its true north.”
But some surgeons said that the technology might nonetheless save money, since patients who receive perfused organs generally leave the hospital quicker and with fewer complications, and have better medium- and long-term outcomes.
Surgeons are still exploring the upper limits of how long perfused organs can survive outside the body, and as substantially as the technologies are already altering transplant, some say this is only the beginning.
Dr. Shaf Keshavjee, a surgeon at the University of Toronto whose lab was at the forefront of developing technologies to preserve lungs outside the body, said the devices could eventually allow doctors to remove, repair, and return lungs to sick patients rather than replace them. “I think we can make organs that will outlive the recipient you put them in,” he said.
Dr. Ashish Shah, the chair of cardiac surgery at Vanderbilt University, one of the busiest heart transplant programs in the country, agreed, calling that “the holy grail.”
“Your heart sucks,” he said. “I take it out. I put it on my apparatus. While you don’t have a heart, I can support you with an artificial heart for a little while. I then take your heart and fix it — cells, mitochondria, gene therapy, whatever — and then I sew it back in. Your own heart. That’s what we’re really working for.”
This article originally appeared in The New York Times.

